Background Patients undergoing hematopoietic cell transplantation (HCT) must cope with immense physical and psychological symptoms during HCT hospitalization, which contribute to a dramatic deterioration in quality of life (QOL). Yet, studies examining pre-HCT coping strategies are limited. We aimed to characterize use of pre-HCT coping strategies, to identify sociodemographic and clinical factors associated with coping strategy use, and to investigate the association of coping strategy use with pre-HCT QOL and psychological distress.
Methods We conducted a secondary analysis of baseline data from a multi-site randomized controlled trial evaluating a supportive care intervention for patients with hematologic malignancies undergoing allogeneic or autologous HCT (NCT#03641378). From 10/2018 - 07/2022, the trial enrolled consecutive adults (age ≥ 18) with hematologic cancers undergoing HCT at three tertiary care academic centers. Prior to HCT, we assessed QOL (Functional Assessment of Cancer Therapy - Bone Marrow Transplant), psychological symptoms (Hospital Anxiety and Depression Scale and Post Traumatic Stress Disorder (PTSD) - Civilian Version), and coping (Brief COPE). We categorized coping as either “approach-oriented” (active coping, use of emotional support, positive reframing, acceptance) or “avoidant” (behavioral disengagement, denial, and self-blame). We used the median split method to categorize high and low use of approach-oriented and avoidant coping. Univariate logistic regression was used to identify sociodemographic and clinical factors associated with high use of coping strategies. Multivariate linear regression was used to measure associations of coping strategy use with QOL and psychological distress.
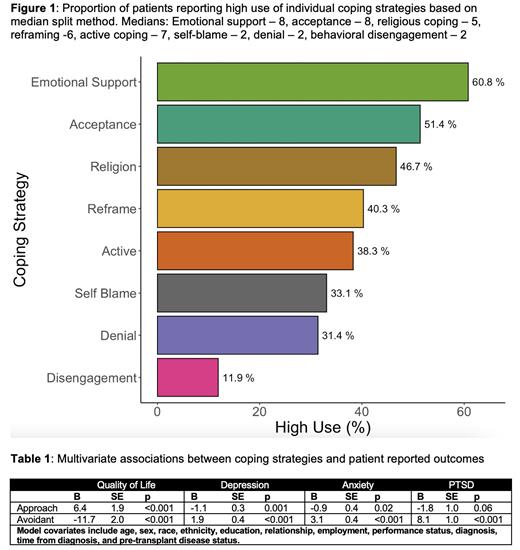
Results Of pre-HCT patients (n=360), the majority were male (62.8%), identified as White (77.5%), were younger than 65 (73.6%), and had received at least some college education (78.9%). A minority were unemployed due to disability from illness (29.2%). Approximately half received autologous HCT (n=178, 49.4%). Figure 1 displays the proportion of patients scoring as high utilizers of individual coping strategies. While 31.3% were high utilizers of avoidant coping, 43.5% were high utilizers of approach-oriented coping. Notably, 43 (11.9%) patients identified as high utilizers of both approach-oriented and avoidant coping. No sociodemographic or clinical factors were associated with use of approach-oriented coping. Older age (>65 years, OR=0.9, p=0.01) and higher income (>$100K, OR=0.90, p=0.04) were associated with less frequent use of avoidant coping. Female gender (OR=1.11, p=0.03) and disabled employment status (OR=1.11, p=0.05) were associated with increased likelihood of high use of avoidant coping. Table 1 displays adjusted associations between approach-oriented and avoidant coping strategies and patient-reported outcomes. High use of approach-oriented coping was associated with better pre-HCT QOL (B=6.4, p<0.001) and lower depression (B=-1.1, p=0.001) and anxiety (B=-0.9, p=0.02) symptoms. High use of avoidant coping was associated with worse pre-HCT QOL (B=-11.7, p<0.001) and symptoms of depression (B=1.9, p<0.001), anxiety (B=3.1, p<0.001), and PTSD (B=8.1, p<0.001).
Conclusions Prior to HCT, patients frequently employ approach-oriented and avoidant coping strategies. Key sociodemographic factors including age, gender, income level, and employment status were associated with avoidant coping. Patients with high use of approach-oriented coping reported higher baseline QOL and lower psychological distress, while those with high use of avoidant coping strategies reported worse QOL and psychological distress. These data suggest that supportive care interventions which enhance approach-oriented coping and mitigate avoidant coping in pre-HCT populations may improve QOL and reduce psychological distress
Disclosures
Newcomb:Vertex: Current equity holder in publicly-traded company, Other: Spouse employment; Timedoc: Divested equity in a private or publicly-traded company in the past 24 months. LeBlanc:Agilix: Consultancy, Honoraria; UpToDate: Patents & Royalties; BlueNote: Consultancy, Honoraria; CareVive: Consultancy, Honoraria; Deverra Therapeutics: Research Funding; Duke University: Research Funding; American Cancer Society: Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; BeiGene: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Dosentrx: Current equity holder in private company; Flatiron: Consultancy, Honoraria; Astellas: Consultancy, Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria, Speakers Bureau; Incyte: Honoraria, Speakers Bureau; Genentech: Consultancy, Honoraria; GSK: Consultancy, Honoraria, Research Funding; Lilly: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Meter Health: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Leukemia and Lymphoma Society: Research Funding; National Institute of Nursing Research/National Institutes of Health: Research Funding; Seattle Genetics: Research Funding; Servier: Consultancy, Honoraria. El-Jawahri:Incyte Corporation: Consultancy; GSK: Consultancy; Novartis: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal